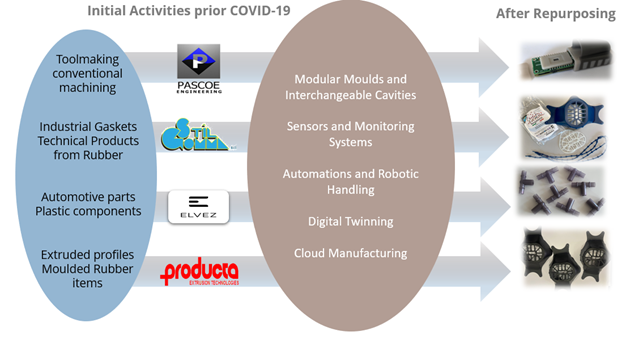
Within the pandemic period of COVID-19 and after 18 months, starting on December 2020, imPURE project developed a methodology for the fast repurposing of Industrial Injection Moulding Lines, through enabling Additive Manufacturing Technologies, in order to manufacture interchangeable cavity inserts, to be used for the production of Critical Medical Supplies.

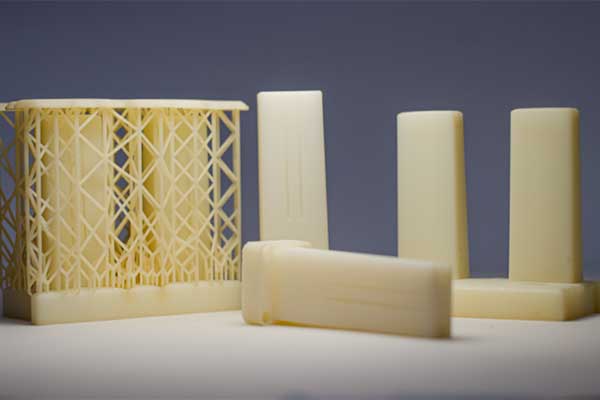
Mould insert manufactured via DED by CONIFY (left) and ceramic sliders manufactured via SLA by BIOG3D (right)

Mould insert manufactured via a hybrid approach using WAAM by IRT JULES VERNE
The following targets were reached:
- Exploitation and repurposing of four existing Injection Moulding lines for the production of Critical Medical Supplies
- Design and manufacturing of four new modular mould sets for Injection Moulding and refurbishing of used moulds, exploiting AM technologies and machining processes
FACE MASKS
Ergonomic Reusable Respirator Silicone Mask designed and produced in the Repurposed IM Line of STILLGOMMA and new Thermoplastic Mask with Antimicrobial Properties for Healthcare Professionals produced in the Repurposed IM Line of PRODUCTA
SPIKE FOR VACCINES
Ergonomic Vaccine Spike for Covid-19 vaccines designed by SIDAM and produced in the Repurposed IM Line of ELVEZ
PULSE OXIMETERS
New design of finger pulse oximeter designed by UOS and NIT and produced in the Repurposed IM Line of PASCOE
VENTILATOR SPLITTERS
T-shape Ventilator Splitter accessory for dual circuit configuration designed by SIDAM and produced in the Repurposed IM Line of ELVEZ
Repurposed IM Lines
The Repurposing of four Injection Moulding Lines was achieved, through the development of modular moulds by the combination of Additive Manufacturing Technologies and Conventional Tooling. Within imPURE, automation technologies and remote monitoring systems were employed for Cloud Manufacturing (CMfg)-based service integration, which were demonstrated in plastic processing and Injection Moulding Lines available in the consortium (PASCOE, STIL, ELVEZ, PROD). Optimal configuration methods were applied based on the degree of automation and monitoring of each line, supported by multi-physics simulation models with feedback from sensors in the production workflow. Various data in the entire injection moulding process, including mould design, mould manufacturing and the injection moulding process were linked to achieve more efficient, smart and sustainable manufacturing environment.
- Critical Medical Supplies including the products’ design, materials used, and crucial operational requirements were defined
- Polymers with antimicrobial properties were prepared incorporating Ag nanoparticles, which were used as modified materials for the production of the CMSs
- Toxicological assessment for potential hazard risks from nanomaterials handling and processing as a general framework of safety use during production line repurposing
- A market overview and a classification of the available metal powders were performed, based on the established in-house metal AM powder characterization workflow, together with specific procedures for refurbishing used AM powders
- A regulatory framework for the exploitation of the products were defined
- An IM production lines’ upgrading/repurposing strategy was developed; the IM lines of PASCOE, STIL, ELVEZ and PROD were repurposed with interchangeable cavity inserts manufactured through AM
- Repurposing strategies included both the design of new modular mould sets and the refurbishment of existing moulds for producing CMSs
- Set of sensors and transducers were installed on IM machines for process monitoring
- Software and hardware tools developed for the integration of data acquisition and communication systems
- A robot handling system was designed, programmed, and applied to the PASCOE IM machine, aiming to enhance production automation, together with an infrared camera for the quality monitoring of the produced parts
- Advanced flow simulations for conformal cooling channels were employed for the optimisation of manufacturing of the mould insert for the ventilator splitter
- A methodology for each of the AM processes, i.e. SLM, DED, Hybrid approach with WAAM was developed for cost-lead time optimisation
- Hybrid ceramic mould components were produced by SLA 3D printing, assessing the required processability and dimensional accuracy
- The Data Management Plan defined the information workflow within the project and possible risks
- User experience research was carried out through interviews with different representatives from the pilot lines, resulting in defining the proto-personas, user journey, and user needs statements to design the command centre for the pilot lines
- A cloud manufacturing platform was developed for the selected pilot examined, and a proof of concept was performed to interact with the Machine Learning models
- A supply chain interactive platform was developed for raw materials and CMSs
- Networking/dissemination activities included more than 150 actions: meetings and video calls with companies, universities, districts, and experts, participation in conferences, workshops, exhibitions, etc.
- A scientific paper was also published, available at: https://doi.org/10.3390/polym14122418
imPURE IMPACT
The impact of imPURE relates as such on applying imPURE methodology to whenever such repurposing is needed, within pandemic situation or even outside of it, for companies and industry sector not only to run flawlessly with no downtime but also to enter new markets.
imPURE impact can be summarised in the following:
The flowchart used in impure is a methodological tool that provides:
Now after imPURE, every industry has access to the steps required to do repurposing!



